Today Tuesday May 7, at 04.00 p.m. CET IRRAS will host a conference call and an online presentation of its Q1 2019 interim report (which was published earlier today 08.00 a.m. CET).
The dial-in numbers for the conference call are:
Sweden: +46 8 5664 2707
Rest of the world: +44 33 3300 9034
The presentation will also be webcast and can be accessed from the following web address:
https://financialhearings.com/event/11740
Hosts: President CEO Kleanthis G. Xanthopoulos, Ph.D. and CFO and Deputy CEO Fredrik Alpsten
Investor and Media Contact:
Fredrik Alpsten
CFO and Deputy CEO
+46 706 67 31 06
fredrik.alpsten@irras.com
About IRRAS
IRRAS AB (Nasdaq First North Premier: IRRAS) is a publicly-traded, commercial-stage medical technology company focused on developing and commercializing innovative solutions for brain surgery.
The company’s initial product, IRRAflow, is the world’s first “irrigating ventricular drain.” Its unique mechanism of action addresses the complications associated with the current methods of managing intracranial fluid by using a dual lumen catheter that combines active irrigation with ongoing fluid drainage. Additionally, IRRAflow incorporates ICP monitoring and uses a proprietary software to regulate treatment based on desired pressure levels. IRRAflow received FDA-clearance in July 2018.
With its unique product portfolio, protected by property patents and patent applications, IRRAS is well positioned to establish a leadership position in the medical device market. IRRAS maintains its headquarters in Stockholm, Sweden, with corporate offices in Munich, Germany, and San Diego, California, USA. For more information, please visit www.irras.com.
IRRAS AB (PUBL)INTERIM REPORT JANUARY–MARCH 2019
First quarter, January – March 2019
- Net revenue totaled SEK 0.0 million (5.9).
- Operating loss (EBIT) amounted to SEK -39.1 million (-21.1).
- Loss after tax totaled SEK -38.7 million (-17.8).
- Earnings per share before and after dilution amounted to SEK -1.61 (-0.75).
Important events during the quarter
First patients in the US successfully treated with IRRAflow®
At the beginning of January, the first patients in the United States were treated with our lead product, IRRAflow. The successful treatments were performed at the University of California– Irvine.
New financial targets
In February the company presented new financial targets. The targets are:
– Revenue exceeding SEK 275 million in 2021
– Gross margin exceeding 72% in 2021
– Cash-flow positive by Q4 2021
Strengthened executive management team
In March, Vinny Podichetty, MD, MS, was hired as VP of Global Clinical Affairs. He is reporting to the President, CEO, and will serve as a member of the executive team.
Evaluates possible listing on Nasdaq Stockholm’s main market*
The company announced its plans to evaluate a possible listing of its share at the Nasdaq Stockholm’s main market in 2019. In addition to Carnegie, IRRAS has also engaged Pareto as a financial advisor.
Important events after the end of the first quarter
Collaboration with AMI-USC
The company entered into an agreement with the Alfred E. Mann Institute for Biomedical Engineering at the University of Southern California (AMI-USC). Under the terms of the agreement, IRRAS will acquire assets and intellectual property from DermaPort, Inc.
Update on CE-mark recertification
The company received a response from G-MED, its Notified Body, that requests clarifications and additional information regarding the CE Mark recertification of the company’s IRRAflow Catheter.
G-MED asked for additional technical clarifications and updates of certain previous older reports performed by the previous Swedish development partner. The requests are part of the routine review cycle and will be addressed by IRRAS in a timely manner.
First sales in US reported
Physician support for IRRAflow has been generated at more than 35 US hospitals. The product approval process is underway at more than 20 hospitals, and almost 10 additional sites are actively evaluating the technology. The feedback thus far has been positive. The first US sales were reported first week of April.
Strengthened position in US by acquiring assets
IRRAS has strengthened its position in the US by acquiring assets of InnerSpace Neuro Solutions. The assets, including four FDA-cleared products, complement IRRAflow and will support IRRAS’ positioning as a leading company in the space. The sales of the new products are expected to start in Q3.
Comments from the President and CEO
Launch in US
The launch in the US continues according to plan. The first patients were successfully treated in January, and the initial treated patients have been followed with great interest by a number of US hospitals. At the moment, we have support from physicians at more than 35 US hospitals, and our commercial team is taking the needed steps for these facilities to evaluate IRRAflow.
In April, AANS (American Association of Neurological Surgeons) held its yearly scientific meeting in San Diego. IRRAS participated and generated significant interest. Our direct sales team is now following up with these leads across the US. In connection with AANS, we had also our first US KOL meeting, which was very well received by the participants.
We believe that the sales process in the US for a new technology takes up to 6 months after initial contact with a new customer before revenue is generated. Our US launch commenced during Q4 2018, and our launch remains on track with the first purchase orders shipped to customers in early April.
Strengthened position in US by acquiring assets, including four FDA-cleared neurocritical care products
We have strengthened our position in US by acquiring the assets of InnerSpace Neuro Solutions. These assets include four FDA-cleared products that complement IRRAflow and will support IRRAS’ positioning as a leading player in the space. We will initiate sales of the acquired products in Q3. The acquired patents and expertise overlap with planned IRRAflow product development and will result in an accelerated timeline and significantly reduced development costs. The acquired products have been used in over 2 000 patients, and their clinical utility is supported by a number of scientific publications.
This acquisition fits perfectly with our strategy to use our innovative fluid exchange technology as a cornerstone to become domain dominant in neurocritical care. We have been following InnerSpace for over a year and are very pleased to complete this transaction. By adding these products to our portfolio, we will use their existing sales channels to introduce IRRAflow to new customers, and our team can now offer US hospitals additional cutting edge products to treat neurocritical care patients. This is a unique opportunity for IRRAS to strengthen our position and become a leading supplier in US, the world’s most important neurosurgical market.
Update received on CE Mark re-certification of IRRAflow
In April, we received the feedback from G-MED after a lengthy period. G-MED has asked for additional technical clarifications and updates of certain previous older reports. The requests are part of the routine review cycle, and we believe that all of their comments are addressable. Having established a productive dialogue with the notified body, we have a clear path forward, and we anticipate responding shortly to the list of questions. We will continue to work closely with the G-MED team to reintroduce this innovative medical device to the EU market, offering patients, neurosurgeons, and hospitals an effective, intelligent solution to treat intracranial bleeding.
First quarter financial overview
The first US sales were reported first week of April. Thus, due to the delay in the recertification of the CE mark in the EU and pending sales in the US, no sales were reported during the first quarter of 2019.
EBIT for the first quarter of the year was SEK -39.1 million (-21.1). The increased costs are primarily attributable to the planned organizational expansion within sales and marketing, production, R&D and administration. The average number of employees in the first quarter of 2019 was 32, compared with 17 in the year-earlier period.
Our available liquidity as of March 31, 2019 amounted to SEK 116 million, including short- and long-term financial investments.
Building for future growth at IRRAS
At IRRAS, our mission is clear: to change the lives of millions by creating medical products that transform the current treatment of intracranial bleedings. We believe that IRRAflow will ultimately become the new standard of care in this field, and, during the quarter, we continued to make progress toward accomplishing this mission.
With the US launch, the anticipated recertification of the CE mark in the EU, our registration of our product in additional countries, and our portfolio of differentiated technology and unique products, we believe that we are setting the foundation to become domain dominant in the neurocritical care market.
President and CEO Kleanthis G. Xanthopoulos, Ph.D.
| Calendar | |
| Q2 2019 Interim Report | August 29, 2019 |
| Q3 2019 Interim Report | November 8, 2019 |
| Annual Shareholders’ meeting |
May 14, 2019 |
Financial information
Kleanthis G. Xanthopoulos, Ph.D, President and CEO
kleanthis.xantopoulos@irras.com
Fredrik Alpsten
CFO and Deputy CEO
+46 706 67 31 06
fredrik.alpsten@irras.com
This document is considered information that IRRAS is obliged to disclose pursuant to the EU Market Abuse Regulation. The information was released for public disclosure, through the agency of the contact person above, on May 7, 2019 at 8:00 a.m. (CET).
IRRAS strengthens position in neurocritical care by acquiring proprietary assets, including four US FDA-cleared products
– Assets complement IRRAflow® and support positioning as a leading company in the space
– Sales of the acquired products will be initiated in the US in Q3
– Acceleration of IRRAS’ product development plans and significant cost savings
Stockholm, May 7, 2019 – IRRAS AB (Nasdaq First North Premier: IRRAS), a medical technology company focused on commercializing innovative solutions for neurocritical care, announced today it has acquired the assets of InnerSpace Neuro Solutions Inc. with immediate effect. These include proprietary single and multi-lumen cranial access bolts, parenchymal ICP (Intracranial Pressure) monitoring, and cranial access kit. The total purchase price for these assets, which are 510(K) FDA cleared in the United States but have not yet received CE Mark, was 700,000 USD.
The InnerSpace products have been used in over 2 000 patients, and their clinical utility is supported by a number of scientific publications. They complement IRRAS’ current IRRAflow product line and substantially expand the company’s available product portfolio. Sales are expected to be initiated by the IRRAS’ US commercial organization in Q3 2019.
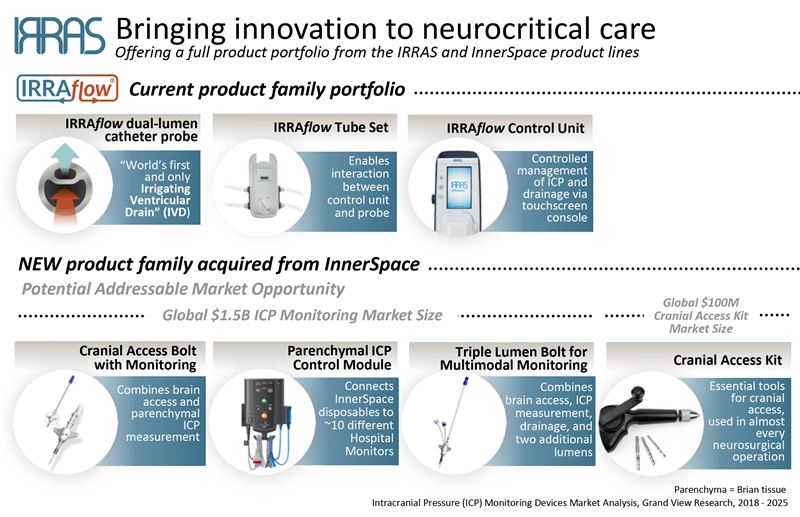
“This is a unique opportunity for IRRAS to strengthen its position and become a leader in the neurocritical care market. These innovative and proprietary products are a perfect fit to our product portfolio. In addition, we will use established InnerSpace sales channels to introduce IRRAflow to new customers,” said Kleanthis G. Xanthopoulos, Ph.D., President & CEO.
There are clear and immediate commercial synergies that will provide IRRAS with innovative solutions across the treatment spectrum in neurocritical care. Furthermore, the acquired patents and expertise will result in accelerated timeline and significantly reduced development costs of planned IRRAflow products.
About IRRAS
IRRAS AB (Nasdaq First North Premier: IRRAS) is a publicly-traded, commercial-stage medical technology company focused on developing and commercializing innovative solutions for brain surgery.
The company’s initial product, IRRAflow, is the world’s first “irrigating ventricular drain.” Its unique mechanism of action addresses the complications associated with the current methods of managing intracranial fluid by using a dual lumen catheter that combines active irrigation with ongoing fluid drainage. Additionally, IRRAflow incorporates ICP monitoring and uses a proprietary software to regulate treatment based on desired pressure levels. IRRAflow received FDA-clearance in July 2018.
With its unique product portfolio, protected by property patents and patent applications, IRRAS is well positioned to establish a leadership position in the medical device market. IRRAS maintains its headquarters in Stockholm, Sweden, with corporate offices in Munich, Germany, and San Diego, California, USA. For more information, please visit www.irras.com.
IRRAS AB (publ) is listed on Nasdaq First North Premier. Wildeco is certified adviser of the company. Wildeco is reached at + 46 8 545 271 00 or at info@wildeco.se.
For more information, please contact:
US
Kleanthis G. Xanthopoulos, Ph.D.
President & CEO
info@irras.com
Europe
Fredrik Alpsten
CFO and Deputy CEO
+46 706 67 31 06
fredrik.alpsten@irras.com
This document is considered information that IRRAS is obliged to disclose pursuant to the EU Market Abuse Regulation. The information was released for public disclosure, through the agency of the contact person above, on May 7, 2019 at 08.00 a.m. (CET).
IRRAS’s nomination committee proposes Ms. Catherine Gilmore-Lawless as new board member
Stockholm, May 3, 2019 – IRRAS AB (Nasdaq First North Premier: IRRAS), a commercial-stage medical-technology company, today announced that the company’s nomination committee recommends Ms. Catherine Gilmore-Lawless to be a new IRRAS board member.
On April 15, 2019, in the notice for the Annual General Meeting, the nomination committee for IRRAS presented its proposals to the AGM, which will be held on May 14, 2019. The notice stated that the nomination committee intended to propose an additional board member to be presented as soon as possible, no later than in connection with the AGM.
Ms. Gilmore-Lawless is an American citizen and has 30 years of experience in the neuroscience sector. Her experience includes more than 15 years in several leading positions at Elekta Instrument AB, including President of the company’s US subsidiary where she was instrumental in introducing new neurosurgical technology including image guidance and radiosurgery to the U.S. market. Other roles at Elekta included Senior Vice President, Marketing and Vice President, Clinical Intelligence, Neuroscience.
Ms. Gilmore-Lawless also served as Chief Development Officer of Neurosource Inc., a neuroscience consulting and practice management firm and CEO of CINN Foundation, a non-profit organization dedicated to improving the lives of individuals afflicted with neurological disorders.
The nomination committee’s assessment is that Ms. Gilmore-Lawless is well suited to be a board member of IRRAS and that she will add valuable additional expertise and experience from the North American neurosurgery market. Ms. Gilmore-Lawless is deemed to be independent both in relation to IRRAS and to the company’s major shareholders.
About IRRAS
IRRAS AB (Nasdaq First North Premier: IRRAS) is a publicly-traded, commercial-stage medical technology company focused on developing and commercializing innovative solutions for brain surgery.
The company’s initial product, IRRAflow®, is the world’s first “irrigating ventricular drain.” Its unique mechanism of action addresses the complications associated with the current methods of managing intracranial fluid by using a dual lumen catheter that combines active irrigation with ongoing fluid drainage. Additionally, IRRAflow incorporates ICP monitoring and uses a proprietary software to regulate treatment based on desired pressure levels. IRRAflow received FDA-clearance in July 2018.
With its unique product portfolio, protected by property patents and patent applications, IRRAS is well positioned to establish a leadership position in the medical device market. IRRAS maintains its headquarters in Stockholm, Sweden, with corporate offices in Munich, Germany, and San Diego, California, USA. For more information, please visit www.irras.com.
IRRAS AB (publ) is listed on Nasdaq First North Premier. Wildeco is certified adviser of the company. Wildeco is reached at + 46 8 545 271 00 or at info@wildeco.se.
For more information, please contact:
Fredrik Alpsten
CFO and Deputy CEO
+46 706 67 31 06
fredrik.alpsten@irras.com
This document is considered information that IRRAS is obliged to disclose pursuant to the EU Market Abuse Regulation. The information was released for public disclosure, through the agency of the contact person above, on May 3, 2019 at 08.00 a.m. (CET).
IRRAS Provides Update on CE Mark Re-Certification of IRRAflow®
Stockholm, April 24, 2019 – IRRAS AB (Nasdaq First North Premier: IRRAS), a commercial-stage medical technology company focused on developing and commercializing innovative solutions for neurocritical care, announced today that it received a response from G-MED, its designated European Notified Body, that requests clarifications and additional information regarding the company’s CE Mark re-certification of IRRAflow Catheter.
G-MED has asked for additional technical clarifications and updates of certain previous older reports performed by the previous Swedish development partner. The requests are part of the routine review cycle and will be addressed by IRRAS in a timely manner.
“We finally received the feedback from G-MED after a lengthy period,” said Kleanthis G. Xanthopoulos, Ph.D, President and CEO of IRRAS. “We believe that all of their comments are addressable. Having established a productive dialogue with the notified body, we now have a clear path forward, and we anticipate responding shortly to the list of questions. We will continue to work closely with the G-MED team to reintroduce this innovative medical device to the EU market, offering patients, neurosurgeons, and hospitals an effective, intelligent solution to treat intracranial bleeding. In the meantime, the launch of IRRAflow in the United States is on track since our 510(k) clearance last year, and our plans to open other global markets remain unchanged.”
About IRRAS
IRRAS AB (Nasdaq First North Premier: IRRAS) is a publicly-traded, commercial-stage medical technology company focused on developing and commercializing innovative solutions for brain surgery. The company’s initial product, IRRAflow, is the world’s first “irrigating ventricular drain.” Its unique mechanism of action addresses the complications associated with the current methods of managing intracranial fluid by using a dual lumen catheter that combines active irrigation with ongoing fluid drainage. IRRAflow received FDA-clearance in July 2018.
Regularly during treatment, the IRRAflow catheter is automatically flushed to prevent common catheter occlusions from forming. Because IRRAflow is a completely closed system, it is designed to reduce the documented infection risk of these procedures. Additionally, IRRAflow incorporates ICP monitoring and uses a proprietary software to regulate treatment based on desired pressure levels.
With its unique product portfolio, protected by property patents and patent applications, IRRAS is well positioned to establish a leadership position in the medical device market. IRRAS maintains its headquarters in Stockholm, Sweden, with corporate offices in Munich, Germany, and San Diego, California, USA. For more information, please visit www.irras.com.
IRRAS AB (publ) is listed on Nasdaq First North Premier. Wildeco is certified adviser of the company. Wildeco is reached at + 46 8 545 271 00 or at info@wildeco.se.
For more information, please contact:
US
Kleanthis G. Xanthopoulos, Ph.D.
President & CEO
info@irras.com
Europe
Fredrik Alpsten
CFO and Deputy CEO
+46 706 67 31 06
fredrik.alpsten@irras.com
This document is considered information that IRRAS is obliged to disclose pursuant to the EU Market Abuse Regulation. The information was released for public disclosure, through the agency of the contact person above, on April 24, 2019 at 08.00 a.m. (CET).
IRRAS publishes the 2018 Annual Report
Stockholm, April 23, 2019 – IRRAS AB (Nasdaq First North Premier: IRRAS), a commercial-stage medical-technology company focused on developing and commercializing innovative solutions for intracranial bleeding pathologies, announced today it has published its 2018 annual report on www.irras.com.
For more information, please contact:
Fredrik Alpsten
CFO and Deputy CEO
+46 706 67 31 06
fredrik.alpsten@irras.com
About IRRAS
IRRAS AB (Nasdaq First North Premier: IRRAS) is a publicly-traded, commercial-stage medical technology company focused on developing and commercializing innovative solutions for brain surgery. The company’s initial product, IRRAflow, is the world’s first “irrigating ventricular drain”. Its unique mechanism of action addresses the complications associated with the current methods of managing intracranial fluid by using a dual lumen catheter that combines active irrigation with ongoing fluid drainage. IRRAflow received FDA clearance in July 2018.
Regularly during treatment, the IRRAflow catheter is automatically flushed to prevent common catheter occlusions from forming. Because IRRAflow is a completely closed system, it is designed to reduce the documented infection risk of these procedures. Additionally, IRRAflow incorporates ICP monitoring and uses a proprietary software to regulate treatment based on desired pressure levels.
With its unique product portfolio, protected by property patents and patent applications, IRRAS is well positioned to establish a leadership position in the medical device market. IRRAS maintains its headquarters in Stockholm, Sweden, with corporate offices in Munich, Germany, and San Diego, California, USA. For more information, please visit www.irras.com.
IRRAS AB (publ) is listed on Nasdaq First North Premier. Wildeco is certified adviser of the company. Wildeco is reached at + 46 8 545 271 00 or at info@wildeco.se.
This document is considered information that IRRAS is obliged to disclose pursuant to the EU Market Abuse Regulation. The information was released for public disclosure, through the agency of the contact person above, on April 23, 2019 at 05.00 p.m. (CET).
IRRAS Establishes Collaborative Relationship with AMI-USC Through Acquisition of Complementary Medical Device Technology
Stockholm, April 23, 2019 – IRRAS AB (Nasdaq First North Premier: IRRAS), a commercial-stage medical-technology company focused on developing and commercializing innovative solutions for intracranial bleeding pathologies, announced today it has entered into an agreement with the Alfred E. Mann Institute for Biomedical Engineering at the University of Southern California (AMI-USC). Under the terms of the agreement, IRRAS will acquire assets and intellectual property from DermaPort, Inc., a development stage company associated with the AMI-USC, that include innovative access technology that is complementary to IRRAS’ core neurocritical care business and designed to improve clinical outcomes and patient experience.
IRRAS will gain exclusive rights to intellectual property related to a medical device product consistent with AMI-USC’s mission to bridge the gap between biomedical innovation and the creation of commercially successful medical products. In addition, IRRAS and AMI-USC will explore and collaborate to develop other related new technologies and products over time.
“Working with a world-class biomedical engineering center like AMI-USC is a great privilege for IRRAS. We are very pleased to enter into this agreement and look forward to finding future opportunities to work together,” said Kleanthis G. Xanthopoulos, Ph.D., President & CEO of IRRAS. “We are committed to be industry leaders with innovative solutions in this field, and these types of partnerships will play a key role for our future plans.”
Jonathan G. Lasch, Ph.D., Executive Director of AMI-USC said, “This relationship is the latest in a series of successful strategic endeavors from AMI-USC. We feel that IRRAS is a high quality medical device company, and we are thrilled to be able to work with their team to expand our mission and continue to advance the most innovative technologies.”
About AMI-USC
The Alfred E. Mann Institute for Biomedical Engineering is a 501(c)(3) organization affiliated with the University of Southern California and was founded in 1998. AMI-USC has the mission of accelerating the commercialization of biomedical technology invented at USC. For development of its biomedical technology, the institute partners with researchers throughout the university, where it is developed to a mature stage, validated, patented, and can be licensed to an industry partner or used for the formation of a start-up company along with external investment.
About IRRAS
IRRAS AB (Nasdaq First North Premier: IRRAS) is a publicly-traded, commercial-stage medical technology company focused on developing and commercializing innovative solutions for brain surgery. The company’s initial product, IRRAflow, is the world’s first “irrigating ventricular drain”. Its unique mechanism of action addresses the complications associated with the current methods of managing intracranial fluid by using a dual lumen catheter that combines active irrigation with ongoing fluid drainage. IRRAflow received FDA clearance in July 2018.
Regularly during treatment, the IRRAflow catheter is automatically flushed to prevent common catheter occlusions from forming. Because IRRAflow is a completely closed system, it is designed to reduce the documented infection risk of these procedures. Additionally, IRRAflow incorporates ICP monitoring and uses a proprietary software to regulate treatment based on desired pressure levels.
With its unique product portfolio, protected by property patents and patent applications, IRRAS is well positioned to establish a leadership position in the medical device market. IRRAS maintains its headquarters in Stockholm, Sweden, with corporate offices in Munich, Germany, and San Diego, California, USA. For more information, please visit www.irras.com.
IRRAS AB (publ) is listed on Nasdaq First North Premier. Wildeco is certified adviser of the company. Wildeco is reached at + 46 8 545 271 00 or at info@wildeco.se.
For more information, please contact:
US
Kleanthis G. Xanthopoulos, Ph.D.
President & CEO
info@irras.com
Europe
Fredrik Alpsten
CFO and Deputy CEO
+46 706 67 31 06
fredrik.alpsten@irras.com
The information was released for public disclosure, through the agency of the contact person above, on April 23, 2019 at 08.00 a.m. (CET).
NOTICE TO ATTEND THE ANNUAL GENERAL MEETING OF IRRAS AB (PUBL)
Notification to attend etc.
Shareholders who wish to attend the AGM must
- be recorded in the share register kept by Euroclear Sweden AB no later than on Wednesday 8 May 2019; and
- notify the company of their intention to attend the AGM at the latest by Thursday 9 May 2019.
Notification to participate in the AGM must be in writing via the booking form available on the company’s website www.irras.com or by e-mail to AGM2019@irras.com. Notification can also be made by telephone at +46 10 211 51 70. The notification shall state name, personal identification number/ company registration number, address, telephone number and number of shares held.
Proxies
Shareholders represented by proxy must issue a written, signed and dated proxy. If the proxy is issued by a legal entity, a certified copy of the valid registration certificate (Sw. registreringsbevis) of the legal entity (or similar document for non-Swedish legal entity) must be attached to the proxy. The proxy may not be older than one year unless it states that it is valid for a longer period of time (the validity of the proxy may not exceed five years).
In order to facilitate the registration, proxies in its original as well as registration certificates and other authorization documents should be sent to the company address, IRRAS AB (publ), Att: Sabina Berlin, Vasagatan 16, SE-111 20 Stockholm, Sweden well in advance before the meeting.
Proxy forms for shareholders who wish to attend the meeting by proxy will be available on the company website, www.irras.com.
Shareholding in the name of a nominee
In order to be entitled to participate in the meeting, shareholders who hold their shares through nominees (Sw. förvaltare) must request a temporary registration of the shares in their own name, with Euroclear Sweden AB. Shareholders who wishes to obtain such registration must contact the nominee regarding this well in advance of 8 May 2019.
Proposal for agenda
- Opening of the meeting
- Election of chairman of the meeting
- Preparation and approval of the voting list
- Approval of the agenda
- Election of one or two persons to approve the minutes
- Determination as to whether the meeting has been duly convened
- Presentation of the annual report and the auditor’s report as well as the consolidated annual report and the auditor’s report on the consolidated annual report
- Statement by the CEO
- Adoption of the profit and loss statement and the balance sheet as well as the consolidated profit and loss statement and the consolidated balance sheet
- Resolution in respect of appropriation of the company’s profit or loss as set forth in the adopted balance sheet
- Resolution in respect of discharge of the board members and the CEO from liability
- Determination of number of board members and auditors
- Determination of fees for board members and auditors
- Election of board members and Chairman of the Board of Directors
- Election of auditor
- Resolution in respect of guidelines for remuneration for senior executives
- Resolution in respect of authorization for the Board of Directors to issue shares and/or convertible bonds
- Closing of the meeting
Proposals by the Nomination Committee
The Nomination Committee has consisted of Christer Hellström, Chairman of the Nomination Committee, appointed by Bacara Holdings Limited, Anders P. Wiklund, Chairman of the Board of Directors of IRRAS AB, Marios Fotiadis, appointed by Lexington Holding Assets Limited (BVI) and Christos Panotopoulos, appointed by F.EX Endotherapy Limited.
Election of Chairman of the Meeting (item 2)
The Nomination Committee proposes that Anders P. Wiklund is elected as Chairman of the Meeting.
Determination of number of board members and auditors (item 12)
The Nomination Committee proposes that the Board of Directors shall consist of five board members elected by the General Meeting. The nomination committee also proposes that the Company shall have one registered auditing company as auditor.
Determination of fees for board members and auditors (item 13)
The Nomination Committee proposes that the total remuneration for the Board of Directors shall amount to SEK 1,360,000, of which SEK 540,000 shall be paid to the Chairman of the Board of Directors and SEK 240,000 shall be paid to each of the other board members elected by the General Meeting and who are not employees of the group. The Nomination Committee proposes that a fee of SEK 100,000 shall be paid to the Chairman of the Audit Committee and the Remuneration Committee, respectively, and that a fee of SEK 50,000 shall be paid to each of the other members of the Committees. The total remuneration of SEK 1,360,000 as set out above assumes that the Audit Committee consists of two members and the Remuneration Committee consists of three members.
The Nomination Committee proposes that the audit fees shall be paid in accordance with approved invoices.
Election of board members and Chairman of the Board of Directors (item 14)
As members of the Board of Directors until the end of the next Annual General Meeting, the Nomination Committee proposes re-election of Anders P. Wiklund, Kleanthis G. Xanthopoulos, Marios Fotiadis, Anita Tollstadius and Eva Nilsagård. The Nomination Committee further intends to propose an additional Board member, who will be presented as soon as possible and no later than in connection with the Annual General Meeting. Board member Saeid Esmaeilzadeh has declined to be re-elected.
The Nomination Committee proposes re-election of Anders P. Wiklund as the Chairman of the Board of Directors.
Election of auditor (item 15)
The Nomination Committee proposes that the registered auditing company KPMG AB shall be re-elected as auditor for the period until the next AGM. KPMG AB has informed that, if KPMG AB is re-elected as auditor, the authorised public accountant Duane Swanson will continue as the responsible auditor. The Nomination Committee’s proposal is recommended by the Company’s Audit Committee.
Proposals by the Board of Directors
Dividend (item 10)
The Board of Directors proposes that the AGM shall resolve not to distribute any dividends for the financial year 2018.
Resolution in respect of guidelines for remuneration of senior management (item 16)
The Board of Directors proposes that the AGM decides on guidelines for remuneration and other terms of employment for senior executives according to the following.
The Company’s starting point is that salary and other terms and conditions shall enable the group to attract and retain qualified management persons at a reasonable cost for the Company. The remuneration for management persons shall be decided in accordance with IRRAS remuneration policy. The remuneration for management persons consist of fixed salary, variable remuneration, pension and other benefits. In order to avoid that the management persons take unnecessary risks there shall be a fundamental balance between fixed and variable remuneration. Furthermore, the annual general meeting in IRRAS may, if so is ordered, offer long-term incentive schemes such as share or share price related incentive schemes.
Each management person shall be offered a market level fixed salary based on the degree of difficulty, responsibilities, experience and performance. In addition, each management person may from time to time, be offered a variable remuneration (bonus) to be paid in cash. The variable remuneration shall be based on clear predetermined and measurable performance criteria and economic results, as well as predetermined individual objectives and business objectives, and shall also be designed to promote IRRAS long-term value creation. Variable remuneration may not exceed 12 months’ fixed salary.
Management persons shall be offered pension terms that are in accordance with market practice in the country where the management persons habitually resides. Non-monetary benefits shall facilitate the work of the management persons and shall correspond to what is considered reasonable in relation to market practice. The fixed salary during the notice period shall, together with severance pay, not exceed 24 months’ fixed salary. Insofar board members who are elected by the General Meeting carry out work in addition to work on the Board of Directors, it shall be possible to remunerate them for such work. The remuneration shall be in accordance with market terms and shall be approved by the Board of Directors.
The Board of Directors shall, before every Annual General Meeting, consider whether or not additional share or share price-related incentive schemes shall be proposed to the General Meeting. It is the General Meeting that resolves upon such incentive schemes. Incentive schemes shall promote long-term value growth. New share issues and transfers of securities resolved upon by the general meeting in accordance with the rules of Chapter 16 of the Swedish Companies Act are not covered by the guidelines to the extent the Annual General Meeting has taken, or will take, such decisions.
The Board of Directors shall be entitled to deviate from the guidelines in individual cases if there are special reasons for doing so.
Resolution in respect of authorization for the Board of Directors to resolve to issue of shares and/or convertible bonds (item 17)
The Board of Directors proposes that the AGM authorizes the Board of Directors to, on one or several occasions during the period until the next AGM, with or without deviation from the shareholders’ preferential rights, resolve on share issues and/or issues of convertible bonds.
The purpose of the authorization and the reason for the deviation from the shareholders’ preferential rights, if any, is to enable the Company to carry out issues of shares and/or convertible bonds in a time-efficient way to finance acquisitions or investments in new or existing businesses. The issuance of shares or convertible bonds under the authorisation shall, in case of deviation from the shareholders’ preferential rights, be made at a subscription price according to the prevailing market conditions at the time of the issuance of the shares and/or convertible bonds. Payment for subscribed shares and/or convertible bonds shall be made in cash, in kind or by way of set-off.
The Board of Directors’ complete proposal will be available as set out in section “Documents” below no later than on 23 April 2019.
A valid resolution by the General Meeting pursuant to the proposal above requires that the resolution be supported by shareholders representing at least two-thirds of both the votes cast and the shares represented at the General Meeting.
OTHER INFORMATION
Number of shares and votes
As per 15 April 2019, the total number of shares and votes in the Company amounts to 24,017,974. Currently, the Company holds no own shares.
Documents
The Nomination Committee’s reasoned statement regarding the proposals relating to the Board of Directors and information regarding the proposed board members are available at the Company’s website, www.irras.com and at the Company’s office at Vasagatan 16 in Stockholm, and will be sent free of charge to shareholders who so request and provide their postal address or email address. The complete proposal pursuant to item 17 above, and the annual report and the audit report, will be available at the Company’s website and at the Company’s office at the address set out above no later than on 23 April 2019.
The shareholders’ right to submit questions
The board and the CEO shall, if any shareholder so requests and the board believes that it may be done without significantly harming the company, provide information regarding circumstances that may affect the assessment of an item on the agenda, circumstances that can affect the assessment of the company’s or its subsidiaries’ financial position and the company’s relationship to other companies within the group as well as the consolidated financial statements. Anyone wishing to submit questions in advance can do so by sending them to the company at the address mentioned above.
Stockholm, April 2019
IRRAS AB (PUBL)
The Board of Directors
NOTICE TO ATTEND THE ANNUAL GENERAL MEETING OF IRRAS AB (PUBL) (PDF)
IRRAS evaluates a possible listing of the company’s share on Nasdaq Stockholm’s main market during 2019
Stockholm, March 13, 2019 – IRRAS AB (Nasdaq First North Premier: IRRAS), a commercial-stage medical-technology company, today announced the company plans to evaluate a possible relisting of its shares from Nasdaq First North Premier to Nasdaq Stockholm’s main market during 2019. IRRAS has engaged Carnegie Investment Bank as financial advisor to explore this potential relisting and to evaluate other strategic financial initiatives.
A transition to Nasdaq’s main market in Sweden is part of the company’s business strategy to become a leading medical technology company and industry leader in the space of intracranial bleedings.
“Applying for a listing of IRRAS’ shares to Nasdaq Stockholm’s main list has been a goal since we were first listed in 2017, and it reflects the significant progress and achievements we have made since that time, including our commercial-stage product pipeline and global sales initiatives,” said President and CEO Kleanthis G. Xanthopoulos, Ph.D. “Our successful clinical cases to date illustrate how using IRRAflow can result in fewer complications, reduce treatment time for the patient, and lower the costs of treatment for hospitals and health care providers.”
The information was released for public disclosure, through the agency of the contact person below on March 13, 2019 at 08.00 a.m. (CET).
For more information, please contact:
US
Kleanthis G. Xanthopoulos, Ph.D.
President & CEO
info@irras.com
Europe
Fredrik Alpsten
CFO and Deputy CEO
+46 706 67 31 06
fredrik.alpsten@irras.com
About IRRAS
IRRAS AB (Nasdaq First North Premier: IRRAS) is a publicly-traded, commercial-stage medical technology company focused on developing and commercializing innovative solutions for brain surgery. The company’s initial product, IRRAflow®, addresses the complications associated with the current methods of managing intracranial fluid by using a dual lumen catheter that combines active irrigation with ongoing fluid drainage. IRRAflow received FDA clearance in July 2018.
Regularly during treatment, the IRRAflow catheter is automatically flushed to prevent common catheter occlusions from forming. Because IRRAflow is a completely closed system, it is designed to reduce the documented infection risk of these procedures. Additionally, IRRAflow incorporates ICP monitoring and uses a proprietary software to regulate treatment based on desired pressure levels.
With its unique product portfolio, protected by property patents and patent applications, IRRAS is well positioned to establish a leadership position in the medical device market. IRRAS maintains its headquarters in Stockholm, Sweden, with corporate offices in Munich, Germany, and San Diego, California, USA. For more information, please visit www.irras.com.
IRRAS AB (publ) is listed on Nasdaq First North Premier. Wildeco is certified adviser of the company. Wildeco is reached at + 46 8 545 271 00, or at info@wildeco.se.
IRRAS Strengthens Executive Team with Addition of Dr. Vinny Podichetty as Vice President of Clinical Affairs
Stockholm, March 11, 2019 – IRRAS AB (Nasdaq First North Premier: IRRAS), a commercial-stage medical-technology company announced today that it has appointed Vinny Podichetty, MD, MS. as Vice President of Global Clinical Affairs. Dr. Podichetty will report to the President and Chief Executive Officer, Kleanthis G. Xanthopoulos, Ph.D., and will serve as a member of the Executive Management team.
Dr. Podichetty brings more than 17 years of experience in clinical, medical and regulatory affairs and global execution of clinical trials for medical devices. He has developed and successfully managed high-profile clinical programs for Class II and Class III products, post-market clinical research studies and regulatory plans for small and established organizations.
Prior to joining IRRAS, he held senior positions in Clinical Affairs at Edwards Lifesciences, MicroVention Terumo and Zimmer Biomet Inc. Dr. Podichetty launched his career in clinical research at the prestigious Cleveland Clinic Foundation in Ohio. Subsequently in his other industry roles, he served in various leadership capacities in program management, clinical affairs, strategic planning and portfolio management with key responsibilities in IDE and early feasibility/R&D study execution. Dr. Podichetty holds a Diploma in Medicine from India and a Master of Science degree from Cleveland State University.
“I am excited to join IRRAS, and ready to work in achieving the company’s mission of developing innovative solutions that transform fluid management in patients. IRRAS’s commercial portfolio and scope of initiatives from a clinical and scientific perspective are impressive, and I see tremendous potential in its product pipeline to provide optimal patient care therapies,” said Vinny Podichetty.
“Vinny is a valuable addition to the IRRAS management team. His expertise and vast array of experiences will be important as we work to expand our clinical evidence and scientific initiatives over the next several years to support our IRRAflow system and accelerate our plans to become a global leader in the world of neurocritical care,” said Kleanthis G. Xanthopoulos, Ph.D., President and CEO of IRRAS.
For more information, please contact:
US
Kleanthis G. Xanthopoulos, Ph.D.
President & CEO
info@irras.com
Europe
Fredrik Alpsten
CFO and Deputy CEO
+46 706 67 31 06
fredrik.alpsten@irras.com
About IRRAS
IRRAS AB (Nasdaq First North Premier: IRRAS) is a publicly-traded, commercial-stage medical technology company focused on developing and commercializing innovative solutions for brain surgery. The company’s initial product, IRRAflow®, addresses the complications associated with the current methods of managing intracranial fluid by using a dual lumen catheter that combines active irrigation with ongoing fluid drainage. IRRAflow received FDA clearance in July 2018.
Regularly during treatment, the IRRAflow catheter is automatically flushed to prevent common catheter occlusions from forming. Because IRRAflow is a completely closed system, it is designed to reduce the documented infection risk of these procedures. Additionally, IRRAflow incorporates ICP monitoring and uses a proprietary software to regulate treatment based on desired pressure levels.
With its unique product portfolio, protected by property patents and patent applications, IRRAS is well positioned to establish a leadership position in the medical device market. IRRAS maintains its headquarters in Stockholm, Sweden, with corporate offices in Munich, Germany, and San Diego, California, USA. For more information, please visit www.irras.com.
IRRAS AB (publ) is listed on Nasdaq First North Premier. Wildeco is certified adviser of the company. Wildeco is reached at + 46 8 545 271 00 or at info@wildeco.se.
This document is considered information that IRRAS is obliged to disclose pursuant to the EU Market Abuse Regulation.The information was released for public disclosure, through the agency of the contact person above, on March 11, 2019 at 08.00 a.m. (CET).